On September 19 2022, the Environmental Protection Administration (EPA) of Taiwan, China released the draft Guidance on Phrase 1 Registration of New and Existing Chemical Substances in accordance with the requirements of the Regulations on Registration of New and Existing Chemical Substances (the “Registration Regulations”), and the draft Guidance for Hazards and Exposures Assessment of Chemical Substances that specifies provisions on the Phrase 1 and 2 registration of new and existing chemical substances. They are now available for download on the registration platform and also for public consultation.
Except the updates made to the articles of the Regulations, the draft “Guidance on Phrase 1 Registration of New and Existing Chemical Substances” newly supplemented the FAQs on registration for registrants’ reference.
The Guidance can be divided into five chapters:
- Chapter I introduced terms and definitions of the registration system;
- Chapter II specified the registration scope and inapplicable circumstances;
- Chapter III stipulated the registration types and specifications for data submission;
- Chapter V presented the registration tools, menus and contents in the registration system; and
- Chapter IV explained the provisions concerning review, management and information disclosure after registration application.
The draft “Guidance for Hazards and Exposures Assessment of Chemical Substances” mainly focused on the hazard and exposure assessment information of the Phrase 2 registration. Registrants can use this Guidance as a reference to evaluate the risks of chemical substances and if it is worth using.
The draft can be divided into eight chapters:
- Chapter I specified the regulations related to the registration system;
- Chapter II briefly introduced the basic concepts and definitions of terms for hazard and exposure assessment;
- Chapter III-VI introduced the physical and chemical properties of chemical substances, and hazard assessment methods for human health, environment, persistence, bioaccumulation and acute toxicity; and
- Chapters VII-VIII stated assessment measures for labor and environmental exposure and the proposed application tools respectively.
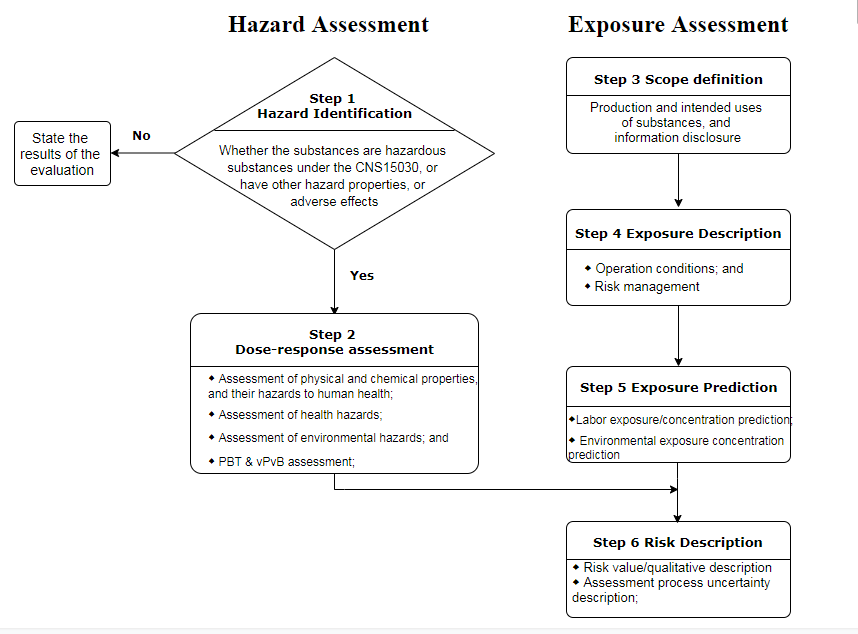
Table 1 Processes for hazard and exposure assessment of chemical substances
As is shown in the Table 1, the whole risk assessment processes are consistent with that under EU REACH. Based on the published guidance documents for risk assessment, evaluation tools such as ECETOC TRA and EUSES are highly recommended. The descriptors of exposure assessment such as ERC and PROC were also introduced into the guidance documents. The parameters of several cases were designed with basic reference to the relevant EU CSR guidance documents. Therefore, if you have completed the risk assessment under EU REACH Regulation, it is not challenging to complete a report under the guidance documents.
If you have any needs or questions, please contact us at service@hfoushi.com.

