According to the "Regulations on the Supervision and Administration of Medical Devices", for newly developed medical devices that have not yet been included in the China Medical Device Classification Catalog, applicants can directly register the products in accordance with the Class III medical device product registration regulations, and it is also possible to determine the product’s classification according to the medical device classification rules or apply to NMPA (NIFDC) for a classification determination. After confirming the product classification, the applicants can apply for medical device registration or filing in accordance with the related regulations.
Service Process:
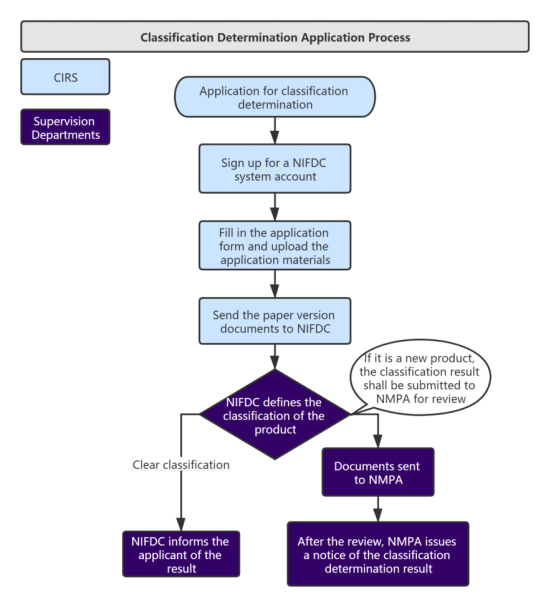
Administrative Fee:
Free
Time Distribution:
NIFDC shall complete the classification determination work within 20 working days from the date of acceptance. If supplementary materials are needed, the applicant shall provide supplementary materials once in accordance with the requirements of the supplementary notice within 30 working days. If the applicant fails to submit supplementary materials as required, or fails to submit supplementary materials within the time limit, NIFDC will return the application. The time required for supplementary information and expert discussions is not included in the time limit.
Related Services:
-
Class I medical device filing and filing change
-
Class II/III medical device registration, registration change and renewal
-
Product testing and rectification technical support
-
Technical files compilation
-
Medical device registration under the MAH system
-
Registration of imported-to-domestic products
-
Follow-up and correction of medical device registration technical review