On January 13, 2023, five countries – Denmark, Germany, the Netherlands, Norway, and Sweden – submitted a restriction proposal to ECHA on per- and polyfluoroalkyl substances (PFASs) under REACH. On February 7, ECHA published the detailed restriction proposal of roughly 10,000 PFASs, which is the broadest restriction proposal in history. Next, ECHA will start a six-month public consultation on PFASs on March 22.
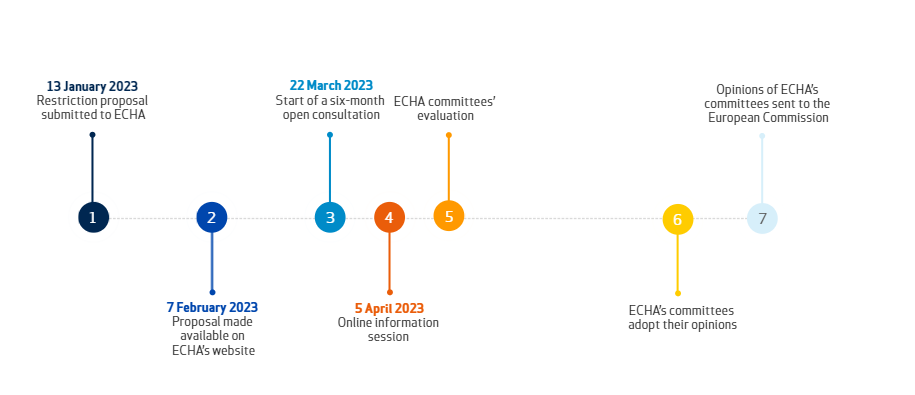
(Fig1) Timeline of PFASs proposal
PFAS substances are widely used in our daily life because they have water, oil, and dirt repellency as well as durability under extreme conditions. To reduce PFAS emissions and make products and processes safer for people, the restriction proposal is made and ECHA’s scientific Committee for Risk Assessment (RAC) will evaluate their potential risks to people and the environment.
In the restriction proposal, the designation of PFASs and conditions of restriction on PFASs are enumerated including the concentration and the date of entry into force, and provisions by way of derogation.
How are PFASs defined?
In the restriction proposal, PFASs are defined as: Any substance that contains at least one fully fluorinated methyl (CF3-) or methylene (-CF2-) carbon atom (without any H/Cl/Br/I attached to it).
A substance that only contains the following structural elements is excluded from the scope of the proposed restriction: CF3-X or X-CF2-X’, where X = -OR or -NRR’ and X’ = methyl (-CH3), methylene (-CH2-), an aromatic group, a carbonyl group (-C(O)-), -OR”, -SR” or–NR”R”’, and where R/R’/R”/R”’ is a hydrogen (-H), methyl (-CH3), methylene (-CH2-), an aromatic group or a carbonyl group (-C(O)-).
The proposed restriction conditions are listed in the following:
- Ban of manufacture, placing on the market, and use;
- PFASs as such, as constituents in other substances or in mixtures as well as in articles;
- Concentration limits are set for 25parts per billion (ppb) for the targeted PFAS; 250 ppb for the sum of PFASs (polymeric PFASs excluded); 50 parts per million (ppm) for PFASs (polymeric PFASs included);
- Transition period: 18 months after entry into force; and
- Use-specific (time-limited) derogations (Additional 5-12 years for the transition periods).
Once the proposal is adopted, the manufacture, placing on the market, and use of PFASs will be restricted. For example, PFAS polymers will also be restricted except for certain uses such as medical apparatus, food contact materials, and fuel cells. Therefore, stakeholders can submit their opinions and express their opinions during the public consultation (six-month duration from March 22).
If you need any assistance or have any questions, please get in touch with us via service@hfoushi.com.
Reference
ECHA News

